
Essay Menu
Acid-Base Indicators - Home Lab Activity #2
All actual activities in this chemistry series must be done with adult supervision!
CheMagic Virtual Molecular Model Kit
Introduction
Acids and bases are important compounds in chemistry. They are common even in the household chemicals we normally use. You are familiar with the sour taste of the acids in lemons and vinegar as well as the pungent odor of ammonia. We do not encourage tasting or smelling of any materials to determine if they are acids or bases. However, there are chemical indicators that can help us to distinguish between acids and bases. Many are dyes; for example, the yellow dye in goldenrod paper is an acid-base indicator. This is illustrated in the video below.
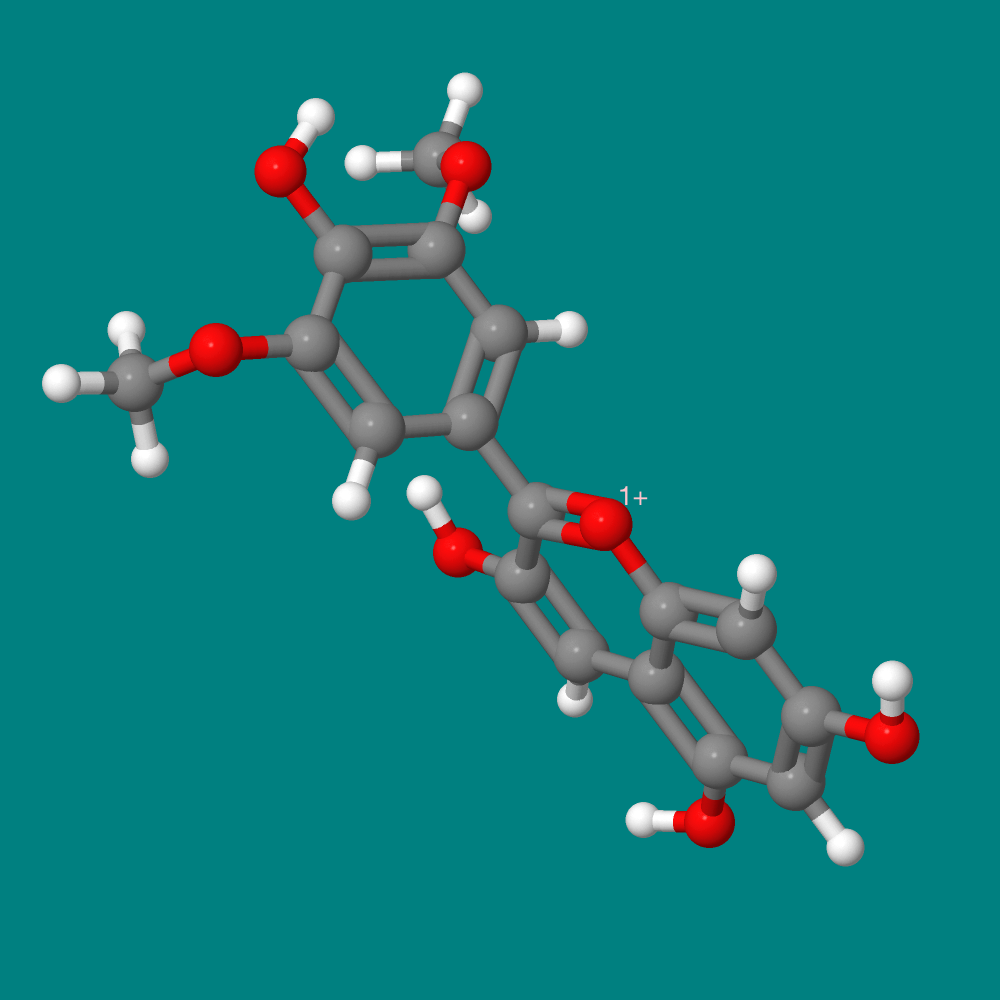
Many acid-base indicators occur naturally. For example, a class of compounds, called anthocyanins, gives berries and plants their red, purple, and blue colors. They act as antioxidants, but they also exhibit different colors in acids or bases. For this experiment, we will be working with the anthocyanins present in grape juice. Most anthocyanins are combined with sugars. The featured molecule in this essay shows the structure of a common grape anthocyanin, malvidin, in its simple form
Experimental Details: Using grape juice as an acid-base indicator
For this experiment, you will need unsweetened grape juice, baking soda, unbuffered aspirin, and vinegar.
Add two ounces of grape juice to one cup of water and mix. Set up three small juice glasses and add four ounces of the diluted grape juice to each.
Add 1/8 teaspoon of baking soda to two glasses and note the color change compared to the third glass.
Add two tablespoons of white vinegar to one glass containing baking soda and note the reaction and color change.
Add five crushed unbuffered aspirin tablets to the other glass containing baking soda.
After a few minutes of stirring, does the aspirin produce an effect similar to the vinegar?
Note that vinegar and aspirin have similar chemical properties in that both are acids. However, they are very different compounds. Vinegar is not a headache remedy!
Based on your observations, propose the result of mixing crushed aspirin with baking soda. Do this experiment by first mixing equal volumes of the solids then adding a few drops of water to the mixture.
Repeat the experiment with other liquids such as, lime juice, lemon juice, orange juice, and household ammonia (you may have to dilute the ammonia to get good results). According to your test results, indicate if they are acids or bases.
Based on your observations, which one of the following pairs are both acids?
| Aspirin & Lime Juice | Baking Soda & Lemon Juice | Ammonia & Lime Juice |
The featured demo video for this page is a classroom demo that used goldenrod paper.
