
Home
Generation of Pure Oxygen - Home Lab Activity #4
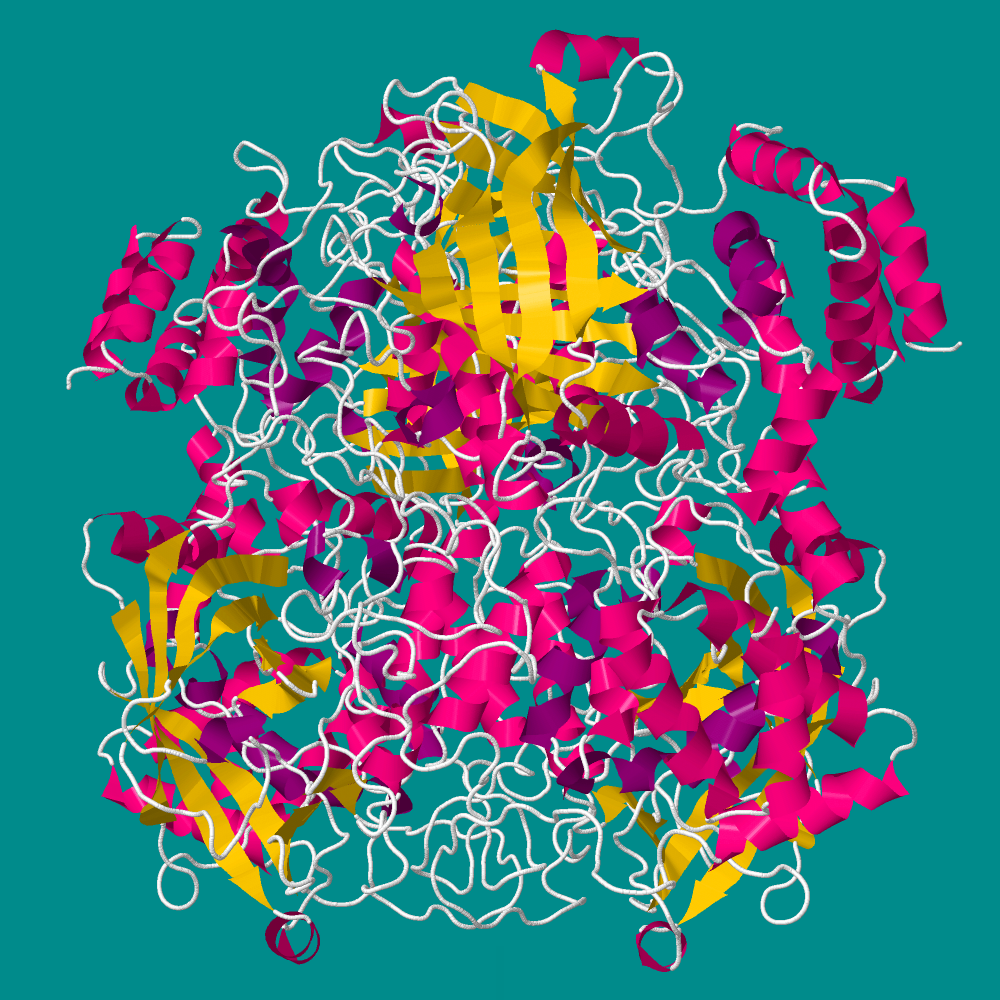
Introduction
We discussed the importance of oxygen in our atmosphere in an essay entitled “Combustion in Oxygen” (https:/chemagic.org/essays).
The generation of oxygen followed by an eye catching combustion demonstration using the oxygen is almost a profession outreach obligation. The featured video below shows our version of this demo in which oxygen is formed by the catalytic decomposition of hydrogen peroxide. This demonstration is similar to the one developed by Hubert Alyea except manganese dioxide is generated in situ.
Generation of pure oxygen showing its ability to support combustion can also be done at home using household materials. Caution: this experiment uses a flame. It should be performed with adult supervision and protective eyewear.
The featured molecule is catalase; it is an enzyme that catalyzes the decomposition of hydrogen peroxide to oxygen and water. This enzyme is found in most organisms that live in the presence of oxygen. It protects cells from damage by peroxidase which is formed by many metabolic reactions.
Experimental Details: Generation of Pure Oxygen
You will need the following household materials: juice glass, piece of cardboard (about 5” by 5”), dried yeast, 3% hydrogen peroxide (do not us a product with a higher concentration), wooden toothpicks, white vinegar, baking soda (sodium bicarbonate).
Add about 2 oz (4 tablespoons) of 3% hydrogen peroxide to a juice glass followed by about ¼ teaspoon of dried yeast. Cover the glass with a piece of cardboard.
Yeast contains an enzyme that causes hydrogen peroxide to decompose into oxygen gas and water
While the hydrogen peroxide is reacting, ignite the end of a toothpick with a match or lighter.
After to reaction has subsided, remove the cover, blow out the flame on the burning toothpick, and insert the glowing toothpick into the glass without immersing it into the liquid.
Record your observations.
Repeat the experiment with 2 oz of white vinegar and one teaspoon of baking soda. In this reaction carbon dioxide is formed.
Does carbon dioxide support combustion?
Our atmosphere consists of about 21% oxygen and 78% nitrogen plus some trace gases as discussed above. If you were to do a similar experiment in which pure nitrogen were generated, would this nitrogen support combustion?
| Yes | No |
The featured demo video for this page is Combustion in Oxygen
