
Essay Menu
Electrolysis - Home Lab Activity #9
All actual activities in this chemistry series must be done with adult supervision!
CheMagic Virtual Molecular Model Kit
Introduction
This experiment will demonstrate electrolysis, a process in which an electrical current is passed through a substance to cause a chemical change.
Electrolysis requires a DC electrical source and an electrolyte to conduct electricity. An electrolyte is a salt that dissolves in water to help carry current through the solution.
In the first part of the experiment, we will evaluate some salts for their ability to act as electrolytes
In the second part of the experiment, we will investigate the electrolysis of water.
Our electrolysis apparatus is a variation of the pencil battery first designed by Kenneth and Doris Kolb (J. Chem. Educ. 1986, 63, 517) consisting of a 9 volt battery and two pencils. The graphite in each pencil will serve as electrodes during the electrolysis.
Oxygen gas is produced at one electrode according to the following reaction:
2H2O --> O2 + 4H+
Hydrogen gas is produced at the other electrode:
2H2O --> H2 + 2OH-
The purpose of the second part of the experiment will be to determine which gas is produced at each electrode.
Experimental Details
You will need: some small juice glasses or cups (4-6 oz), 2 pencils, 9 volt battery, 2 copper wires (6-8 inches) with alligator clips at each end, Epsom salts (MgSO4), table salt (NaCl), baking soda (NaHCO3), red cabbage leaves, distilled water, tap water, household ammonia, vinegar. Wires and alligator clips can be purchased in any hardware store.
The pencil battery is prepared by cutting a notch about 2 cm down from the eraser end of two sharpened pencils exposing the graphite. Attach an alligator clip to the graphite portion of one pencil and the other end to the – terminal of the battery. Do the same for the + terminal and the other pencil (Figure 1).
(Figure 1) 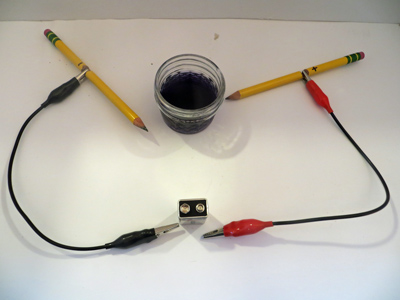
Electrolyte Study:
Set up 5 cups. Add ½ cup of tap water to 4 of the cups, and ½ cup of distilled water to the 5th cup.
Add about 1 teaspoon of Epsom salts, table salt and baking soda to separate cups containing tap water. Mix each thoroughly to dissolve the salts.
Immerse the pencils tips to one of the cups and observe the reaction by noting the production of bubbles (Figure 2). Enter your observation in the table below. Repeat for all of the cups. Use caution when observing the NaCl solution; small amounts of chlorine gas are produced.
(Figure 2) 
DATA TABLE I
| Electrolyte | Observation |
|---|---|
| MgSO4 | |
| NaCl | |
| NaHCO3 | |
| Tap Water | |
| Distilled Water |
Which of the above produced the most bubbles and vigorous reaction?
Which produced the least (or no) reaction?
Electrolysis of Water:
This part of the experiment requires a universal pH indicator to observe the pH at each electrode.
This indicator can easily be prepared by adding about 4 oz of red cabbage leaves to 2 cups of water. Boil the mixture then let sit until it cools. Isolate the liquid portion using a sieve or coffee filter.
Test the indicator by adding a few drops of household ammonia to an ounce of indicator. Retest a fresh ounce of the indicator with a few drops of vinegar. Note the color changes. You may have to dilute the indicator with water to observe a definitive color change.
Add 1 teaspoon of MgSO4 to ½ cup of the pH indicator extract. Insert the two pencil tips of the pencil battery into the solution and note any color change at each electrode. You may have to experiment with the amount of MgSO4 and/or indicator to get bubbles and a definitive color change at each electrode.
Data Table II
| Test | Observation |
|---|---|
| Color of indicator extract | |
| Color of indicator after adding base (ammonia) | |
| Color of indicator after adding acid (vinegar) | |
| Color of indicator at - electrode | |
| Color of indicator at + electrode | |
| pH at - electrode (acidic, neutral, basic) | |
| pH at + electrode (acidic, neutral, basic) |
From your observations and the information in the introduction, which gas is produced at the - electrode?
| Hydrogen | Oxygen |
Which gas is produced at the + electrode?
| Hydrogen | Oxygen |
Red cabbage is a good universal pH indicator because it contains several anthocyanins which are pH sensitive. A water extract of red cabbage exhibits different colors over a range of pH’s. Cyanidin (our featured molecule) and its several glucose derivatives is one anthocyanin found in red cabbage.