
Home
Combustion in Oxygen - Demo Video #2
Introduction
Oxygen is about 20% of the earth’s atmosphere and 50% of the earth’s crust. Consequently, combustion is an important reaction on this planet. It’s no wonder that scientists for centuries have been trying to arrive at a theory of why things burn. For example, Phlogiston theory was an attempt to explain combustion and was popular during the seventeenth and eighteenth centuries.
Antoine Lavoisier proposed the modern theory of combustion in 1777 after Joseph Priestly published his discovery of oxygen in 1774. Think about that! Arguably, the primary chemical reaction process on our planet, combustion, was not fully understood until 1777.
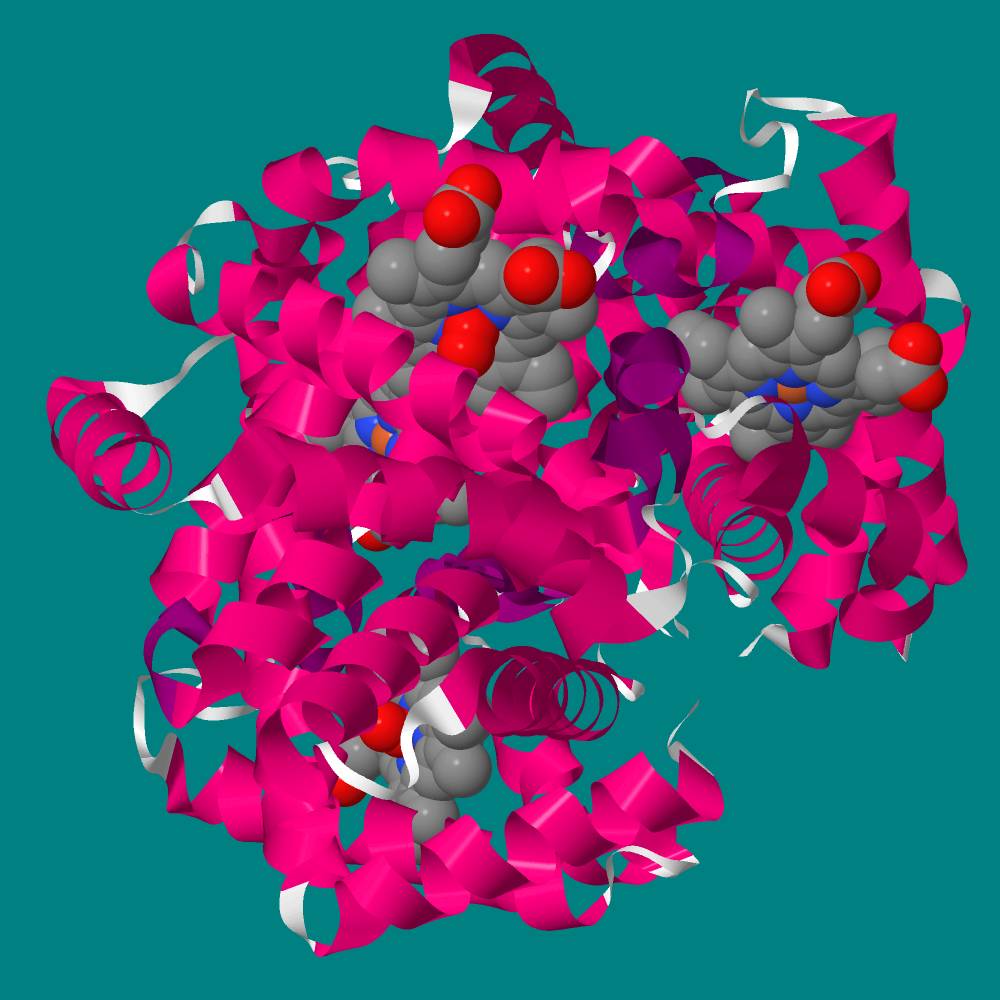
We require oxygen for our “personal” combustion; the average adult uses about 550 liters per day. The featured molecule in this essay is human hemoglobin which is responsible for carrying oxygen from lungs to cells.
Almost every element in the periodic table undergoes some form of oxidation. Metals form oxides that are basic in water while non-metal oxides are acidic in water.
Watch the Basic Blues Demo Video:
