| Tablet Zoom Level Info | -2 | -1 | 0 | +1 | +2 |
| CheMagic Stories and Model Kit Action Pages Return to Model Kit |  Info |
Click the Reggie Red Bird Info icon for an explaination of Model Kit Action Pages. The Info screen also has a link for making your own Action Pages.
The Molecule We Never Made
Many of us use Bromochlorofluoroiodomethane models and drawings to exemplify principles of stereochemistry. The compound, however, resists all attempts at its actual synthesis. It's a pretty little molecule and an effective teacher, so many of us use it. PubChem, of course, has no problem listing hypothetical compounds. In this story's Model Kit action page, you will load this model from PubChem, look up it's PubChem page, convert it to Bromochlorofluoromethane, which is a known compound, and load the known compound's Wikipedia page: Open Action Page
The Molecule That Changed our Ways
Thalidomide was a multi-use drug that was put on the market in Germany in 1957. One of its uses was to treat pregnancy morning sickness. It did not take long to see the connection between this drug and birth defects. There were also other side effect issues with the drug. In the US, FDA pharmacologist Frances Oldham Kelsey did not approve the drug for marketing and distribution. Through due diligence, she picked up on drug company testing issues, and the thalidomide problem in the US was not as severe as in Europe. Kelsey was awarded the President's Award for Distinguished Federal Civilian Service by President John F. Kennedy in 1962. Thalidomide was marketed as the racemate, and it was believed that only one enantiomer was responsible for the side effect. I don't think this was ever proven. The problem is that the human body easily racemizes thalidomide. The action page for this story deals with the chiral carbon in thalidomide: Open Action Page
Two Molecules with a Pleasant Smell
Limonene is a molecule of industrial interest in Bradenton, FL. It's found in orange peels, and when you make orange juice, you have to do something with the orange peels. Orange peels are processed for use in a number of ways, but limonene is a double use molecule. It has odor industry value by itself, but it is also easily converted to carvone, found in spearmint and caraway - Rothenberger, Otis S.; Krasnoff, Stuart B.; Rollins, Ronald B. (1980). "Conversion of (+)-Limonene to (-)-Carvone: An organic laboratory sequence of local interest". Journal of Chemical Education. 57 (10): 741. The action page for this story deals with the chiral carbon in these molecules: Open Action Page
The Molecule That Does Not Want to Be a Molecule
Acetylene has a standard enthalpy of formation of approximately +227 kJ/mol. At high pressures, it is unstable, and it will explode. Explosion products at high pressure are simply carbon and hydrogen. Acetylene's combustion temperature at normal atmospheric pressure is quite high, hence its utility in welding. Because of acetylene's tendency to explode at high pressure, acetone is included in high pressure storage tanks to reduce the pressure by dissolution. A demo video of the combustion and explosion of acetylene is included the CheMagic Demo Video pages The action page for this story deals with bond order change, the Length button and the Optimize button: Open Action Page
Chemistry Double Speak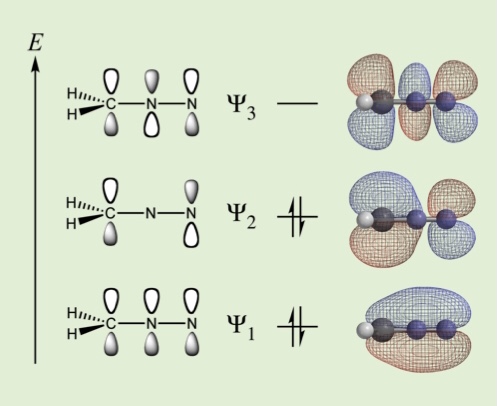 With MO theory as the tool, / This model is a compact jewel. / Its bonds and charges we can "view" / Wrapped up in math with no ado. / But with our eyes we like to see, / Models described by history. / So we oft use another path; / With resonance, we dodge the math. OK, I know resonance theory does not dodge the math, but in reality, that's exactly how most of us use it in our teaching! The inset photo shows the bonding MOs for diazomethane. The action page will show one of three resonance structures for diazomethane. Using the model kit to convert this resonance structure to another resonance structure can get some conversion help from the NCI/CADD Chemical Identifier Resolver. The action page for this story deals with bond order change, atom charge change, and an interesting use of the Chemical Identifier Resolver: Open Action Page
With MO theory as the tool, / This model is a compact jewel. / Its bonds and charges we can "view" / Wrapped up in math with no ado. / But with our eyes we like to see, / Models described by history. / So we oft use another path; / With resonance, we dodge the math. OK, I know resonance theory does not dodge the math, but in reality, that's exactly how most of us use it in our teaching! The inset photo shows the bonding MOs for diazomethane. The action page will show one of three resonance structures for diazomethane. Using the model kit to convert this resonance structure to another resonance structure can get some conversion help from the NCI/CADD Chemical Identifier Resolver. The action page for this story deals with bond order change, atom charge change, and an interesting use of the Chemical Identifier Resolver: Open Action Page
A Molecule Named BOB
SMILES is an acronym for simplified molecular-input line-entry system. The Jmol viewer of the model kit always "knows" the SMILES of the model in the Jmol window. This is very useful in cheminformatics. By default the model kit Name button loads molecules from the PubChem database by actual compound name. The Name button can also be used to load molecules from the NCI/CADD Chemical Identifier Resolver by name or SMILES. This is done by simply starting the name or SMILES with a $ character. The action page for this story deals with use of the Chemical Identifier Resolver to load molecules by SMILES. It also illustrates the cheminformatics power of the model kit using a molecule named BOB!: Open Action Page
A Molecular GoogleWhack
Jacobus van't Hoff and Joseph Le Bel's predicted the chirality of substituted allenes in 1874. Although we often use simple allenes like penta-2,3-diene to illustrate this, the first synthesis to prove the van't Hoff Le Bel hypothesis was of the poor forgotten molecule in this story. If you do a Google search from our model kit (InChI search), you will get a GoogleWhack (single hit search) destroyed by one other hit - my story bragging about finding it in 2017. Technically, a GoogleWhack requires two search words, so I called this an InChI GoogleWhack. Alas, GoogleWhacks are destroyed when you brag about them on the Web. Google just indexes your bragging story - GoogleWhack poof! The synthesis of our forgotten molecule was done in 1924 by Maitland and Mills, Nature 1935, 994. There was no resolution of enatiomers. The proof was simply that the synthesis reaction mixture showed a small optical activity. The action page for this Story deals with use of the JSME drawing app and the Mark-Stereo button. It also illustrates the cheminformatics power of the model kit: Open Action Page
Platonic Molecule Number 1
Tetrahedrane has not yet been synthesized, but the t-butyl substituted tetra-tert-butyltetrahedrane was synthesized in 1978 (Maier, G.; Pfriem, S.; Schäfer, U.; Matusch, R. - Angew. Chem. ). If you attempt to use the model kit to convert tetrahedrane into tetra-tert-butyltetrahedrane, you will become very frustrated. The atomic clutter that creates this frustration may well result in real world steric hinderance that holds tetra-tert-butyltetrahedrane together! The action page for this story provides instructions for using the JSME Molecular Editor that is part of the model kit to convert tetrahedrane into tetra-tert-butyltetrahedrane with very little frustration. The drawing can then be loaded into the model kit for some cheminformatics fun: Open Action Page
Platonic Molecule Number 2
In nineteen-hundred-sixty-four, / Your chemistry we could explore. \ In Illinois, you first appeared, / And chemists of the wold all cheered. / It had been thought you were to strained / For your structure to be retained. / You were the first one to be found, / Platonic alkane that was bound. Cubane is, of course a cycloalkane. It is with poetic license that I use the word "alkane" in my verse. Cubane was synthesized in 1964 by Philip Eaton at the University of Chicago ( Eaton, Philip E.; Cole, Thomas W., J. Am. Chem. Soc. 86 (5): 962–964). It is the first Platonic hydrocarbon to be isolated. The very strained molecule seems to lack kinetic pathways to decomposition. It is the most dense hydrocarbon (1.29 g/cm3) - compare octane (0.703 g/cm3). The action page for this story will lead you to the Wikipedia entry for cubane. Take a look at its physical properties. I think you will be surprised: Open Action Page
A Molecule That Changed its Ways
In 2018, Frances Arnold was awarded the Nobel Prize in Chemistry for her work in the area of directed evolution. She was the first American woman to receive the award. The action page for this story explores the enzyme directed evolution of Protein Data Bank 4kqw into the new enzyme 4kqwx. The directed evolution of this enzyme allowed the organism to carry out the transformation of glucose to isobutyl alcohol with great efficiency. This directed evolution switched the enzyme cofactor preference from NADPH to NADH.The Virtual Molecular Model Kit is a small model tool, but it can explore pdb protein files. The scripting is a bit complex, so the action page for this story will use some magic links! I am not a Jmol pdb file pro, so my magic links will be fairly primitive: Open Action Page
This Molecule is Really a Pro
Felix Hoffmann and Arthur Eichengrün were both Bayer chemists with Hoffmann in a senior position. Eichengrün probably did the first synthesis, but the question of idea credit is muddied by the horror of WWII. Arthur Eichengrün was Jewish and a concentration camp survivor. Hoffmann claimed full idea credit, and Eichengrün disagreed. We will probably never know the full truth. On the issue of synthetic prodrug history, a statement attributed to Hoffmann indicated that aspirin was intended as a precursor to salicylate ion after ingestion. It thus minimized the large dosage stomach irritation of the drug sodium salicylate that was used at the time as a pain drug. The action page for this post illustrates some atom and bond edit buttons. It also works with charge and cheminformatics: Open Action Page
Sweet Dreams Are Made of This
Propofol is used as an anesthetic for minor medical procedures and as a precursor to stronger anesthesia. I recently experienced its affects for the first time during a minor medical procedure, and my verse pretty much sums up the experience. There have been cases of propofol abuse, including one very famous case. The answer to my chemical question is, of course, Friedel-Crafts reaction. What surprised me was the this compound was evidently not isolated and studied until 1977. So what's the problem? The para position has to be blocked, and that evidently delayed the study of this compound. Completely Useless Info: The Eurythmics release Sweet Dreams about six years after propofol was marketed. It's an interesting song about life. The action list for this post illustrates the drawing of propofol using the JSME Editor and loading it into the model kit. It also illustrates an advanced cheminformatics Google search: Open Action Page