
Essay Menu
Gold Penny - Home Lab Activity #10
All actual activities in this chemistry series must be done with adult supervision!
CheMagic Virtual Molecular Model Kit
Introduction
The “Gold Penny Experiment” is a popular demo/experiment in which a copper U.S. Penny is “converted” to gold. The demonstration involves heating a penny immersed in a solution of powdered zinc and sodium hydroxide. After a few minutes, a coating of Zn occurs on the surface of the penny. The penny is removed from the solution and heated forming a “gold” color.
There are three separate chemical reactions that occur in this process:
1. Zinc metal dissolves in the base forming the zincate ion, [Zn(OH)4]-2. Formation of the zincate ion facilitates the reduction of the Zn ion.
2. Zincate is reduced by the copper surface on the penny forming a layer of zinc metal.
3. Upon heating, the copper and zinc layers form the alloy, brass, that is gold in color.
Unfortunately, due to the pyrophoric nature of powdered zinc, this is not a safe experiment to do at home or in a class situation.
We have made two modifications in this experiment to make it safe and easier to do at home.
First, we replaced the Zn metal with a zinc salt (e.g. zinc acetate or the zinc found in zinc supplement tablets). Second, we electroplate the zinc metal onto the penny using the pencil battery described in another experiment .
In addition to serving as a “golden” memento of your chemistry experience, this experiment also illustrates the process of electroplating and the formation of an alloy.
Electroplating is done to protect metals against corrosion and wear, and it is used to improve the appearance of metals as in the production of jewelry. Chromium, silver, and gold are metals commonly used in electroplating. Electroplating involves the application of a voltage across two electrodes immersed in a salt solution (electrolyte). The metal being plated is connected to the negative terminal (cathode) of the power source, and the electrolyte contains ions of the metal to be deposited (e.g. silver ions).
An alloy is the combination of a metal and other substances to alter the properties of the metal. For example, steel is an alloy of iron and carbon which gives iron more toughness and corrosion resistance. Alloys can also be a combination of metals. In this experiment, we will make brass which is an alloy of copper and zinc. Bronze is an alloy of copper and tin.
Experimental Details
You will need the following materials: Pencil battery (refer to Home Lab Activity #9 for instructions, a piece of aluminum foil (~6 inches x 6 inches), U.S. copper pennies, zinc supplement tablets (as zinc gluconate), steel wool, toothpicks, medicine dropper, forceps, lye (sodium hydroxide (NaOH); lye can be purchased at a local hardware store. Lye is used for making soap. Be careful when handling NaOH; it is very caustic. Use eye protection and gloves. Clean up any spills immediately with copious amounts of water.)
Crush three Zn-supplements tablets and add 1/8 cup (30 mL) warm water. Stir the mixture then filter through a coffee filter. Assuming all of the Zn-gluconate is dissolved, the Zn concentration should be about 0.08 M. Alternatively, you can prepare a 0.1 M solution of any available zinc salt.
Dissolve ½ teaspoon of lye in two fluid ounces of water. This solution is about 1 M NaOH
Procedure:
Clean two pennies with steel wool and place them on the Al foil.
Add 10 drops of the Zn solution to the surface of one penny; be careful not to allow the liquid to overflow the penny surface on to the Al.
Add 5 drops of the Zn solution and 5 drops of the NaOH solution to the surface of the second penny. Carefully mix the solutions with a toothpick.
Using the pencil battery apparatus, contact the negative terminal of the battery to the Al foil and the positive terminal to the solution on the penny surface containing just the Zn solution. Do not allow the positive terminal to touch the penny. See Figure 1.
(Figure 1) 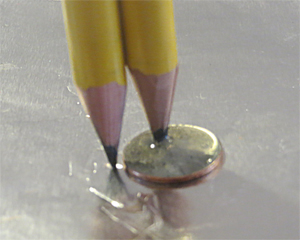
Allow the electroplating to occur for one minute.
Repeat the electroplating on the penny containing the Zn and NaOH solutions.
Carefully remove the pennies with the forceps and wash the pennies with tap water.
Dry them on a paper towel.
Describe the surfaces on the two pennies. Record your observations below.
Using the forceps to hold the penny from the zinc-NaOH electrolyte, gently heat the penny in a flame or on a hot surface. Caution: do not overheat the penny; the inner zinc layer may melt.
Allow the penny to cool.
Describe the change that occurred on the surface of the penny.
DATA TABLE
| Description of Penny Surfaces After Electroplating | Observation |
|---|---|
| Penny From Just the Zn Solution | |
| Penny From the Zn-NaOH Solution | |
| Description of the Penny Surface After Heating |
Discussion:
Which electroplating solution composition resulted in the best deposition of the zinc metal on the penny surface?
What was the role of the sodium hydroxide solution in the mixed solution electrolyte?
The rather startling color change on the surface of the heated penny is due to
| formation of the alloy bronze. | formation of the alloy brass. | electroplating of zinc metal. |
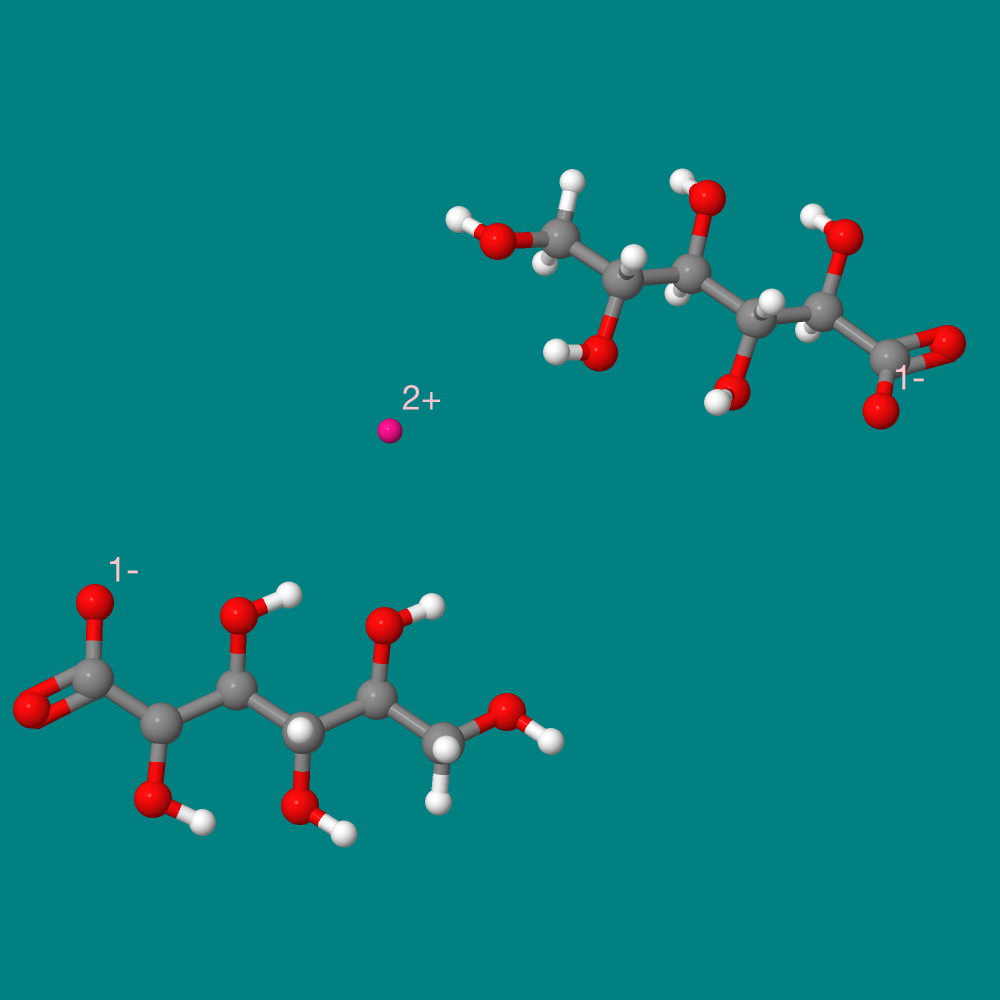
The featured molecule in this experiment is zinc gluconate. It is the salt of one zinc ion and two gluconate acids. It is one of the most common over-the-counter forms of zinc.
At the risk of repeating a video, watch this neat demonstration of another way to illustrate electrolysis with a 9-volt battery.