
Home
Measurement of Oxygen in Air - Home Lab Activity #11
Introduction
We demonstrated oxygen combustion in another home activity and the importance of oxygen in a demo video essay. Although the current concentration of oxygen in the atmosphere is about 21%, this has not always been the case in the history of our planet.
The early earth atmosphere had practically no oxygen. However, due to the persistence of photosynthesis, oxygen levels began to increase about 2 billon years ago. Approximately 300 million years ago the oxygen level increase to a maximum of 35%. This high concentration contributed to large sizes of insects and amphibians. Would you believe dragonflies with a 2.5 ft. wing span?
Oxygen in the atmosphere can be measured by a variety of sophisticated techniques such as chromatography and spectroscopy. However, we can obtain an approximation of the percent oxygen using items found in the home. The basis of the measurement is to follow the rusting of steel wool in an enclosed container inverted in a reservoir of water. As the steel wool combines with oxygen, its depletion causes the water level in the container to rise. The rise in water is proportional to the volume of oxygen used in the rusting process.
Experimental Details
For this experiment you will need steel wool soap pads (They can be popular brands such as SOS®, Brillo®, or your local store brand) and vinegar. You will also need to assemble an apparatus consisting of a water reservoir and a water glass (see Figure 1). When inverted, the glass should just be about 2 cm below the water level. One of the objectives of this activity is to assemble the apparatus from items in your own kitchen.
(Figure 1) 
Procedure:
Rinse the soap from the steel wool pad and soak the pad in vinegar for 10 minutes.
Insert the pad into the bottom of the glass. Be sure the pad does not fall when the glass is inverted (duct tape helps).
Invert the glass into the reservoir; the level of the water in the glass should be the same as the water level outside the glass.
Measure the distance from the water level to the pad. This distance is related the volume of air in the glass.
Measure the increase in the water level in the glass every 5 minutes until no change in the water level is observed.
Record the cumulative time in minutes and the water level in centimeters.
The acetic acid in vinegar acts as a catalyst to increase the speed of the reaction. Repeat the experiment by rinsing the soap from a fresh pad, but do not soak the pad in vinegar.
Discussion:
It is often advantageous to graph data; in this case, to observe how the water level changes with time. Use the following link to graph your data: https://chemagic.org/essays/GCMchart.html
Plot the water level on the Y-axis and the cumulative time on the X-axis. Start the plot at zero time.
Describe the shape of the curve. Is it a straight line?
Calculate the % oxygen from the equation: % = (a/b)100, where b = distance from the water level to the bottom of the pad at the beginning of the experiment and a = the maximum distance the water level rose in the glass.
How does this value compare to the actual % oxygen in air?
What assumptions did you make in the experiment to account for any error?
What was the effect of not soaking the pad in vinegar?
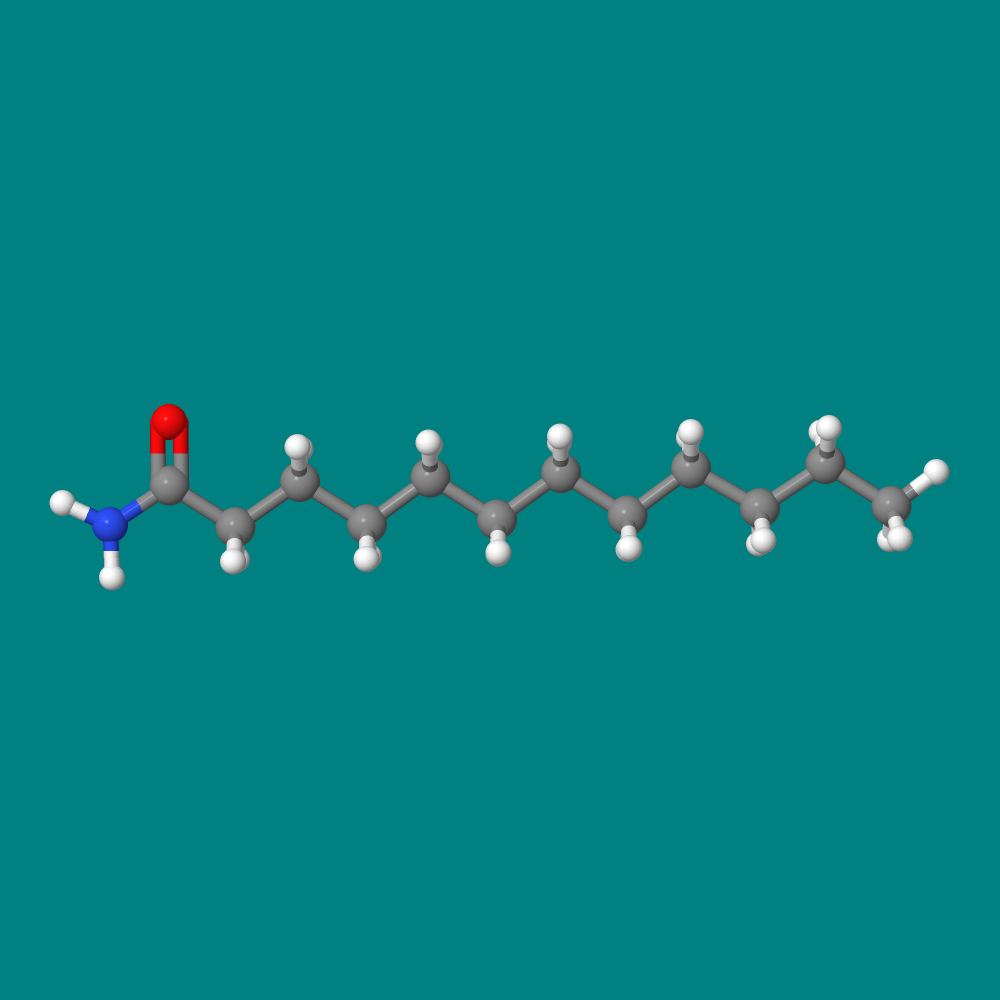
Soap pads are effective because they contain an abrasive (steel wool) and a surfactant to dissolve grease. Our featured molecule is lauramide the main ingredient in cocoamide, the surfactant in Brillo®.
Rusting of steel (a form of combustion) is a relatively slow process; however, in some reactions, combustion can be very rapid and explosive. Our featured video shows the combustion of acetylene.
